Statin pharmacogenomics
Statins are the most commonly prescribed class of drugs used for the prevention and treatment of cardiovascular disease, and function primarily through their ability to lower LDL cholesterol. However, the magnitude of this effect varies widely among individuals, with nearly a third of individuals failing to meet their lipid lowering goals on treatment, and nearly two-thirds of heart attacks occuring on treatment. In addition, some individuals develop adverse effects from statin treatment including new-onset diabetes and statin-induced myopathy. Our objective is to identify molecular and genetic determinants of inter-individual variation of statin response, in terms of both efficacy and adverse effects. Toward this goal we are using a combination of approaches including genetic association studies in clinical trials and population-based cohorts, transcriptomic and metabolomic analyses of cellular statin response using patient-derived iPSCs from a cohort of statin users with varying response, as well as systems biology based approaches to combine and integrate omics-level datasets from our cellular and clinical cohorts. The results of this research could inform the development of precision medicine standards to identify those individuals most likely to achieve cardiovascular benefit from statin treatment versus those who may be at high risk for adverse outcomes.
Disease modeling using iPSCs
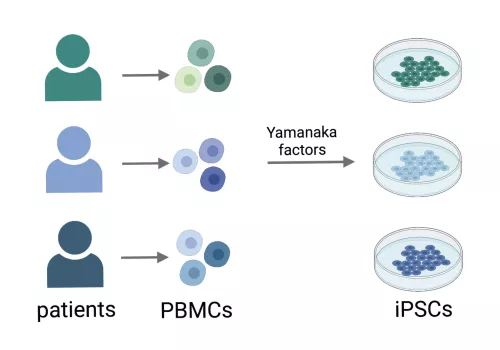
Induced pluripotent stem cells (iPSCs) are generated by reprogramming a somatic cell into a pluripotent stem cell through the heterologous expression of transcription factors, known as Yamanaka factors.
The major benefit of iPSCs is that these cells can be made from specific individuals where they retain the genetic characteristics of the donor. Unlike primary cells, patient-derived iPSC exhibit self-renewal and can be cryopreserved, rendering them to be a highly versatile cellular model.
We have created a repository of iPSCs from individuals at high risk for cardiometabolic diseases including type 2 diabetes and nonalcoholic fatty liver disease (NAFLD), to which we are applying genomic, transcriptomic and functional measures to identify molecular factors that underlie individual-level differences in disease risk.
Phosphatidylinositides and lipid metabolism
As key directors of intracellular trafficking, phosphotidylinsotides (PIs) are a family of low-abundance lipids that impact almost every process within the eukaryotic cell.
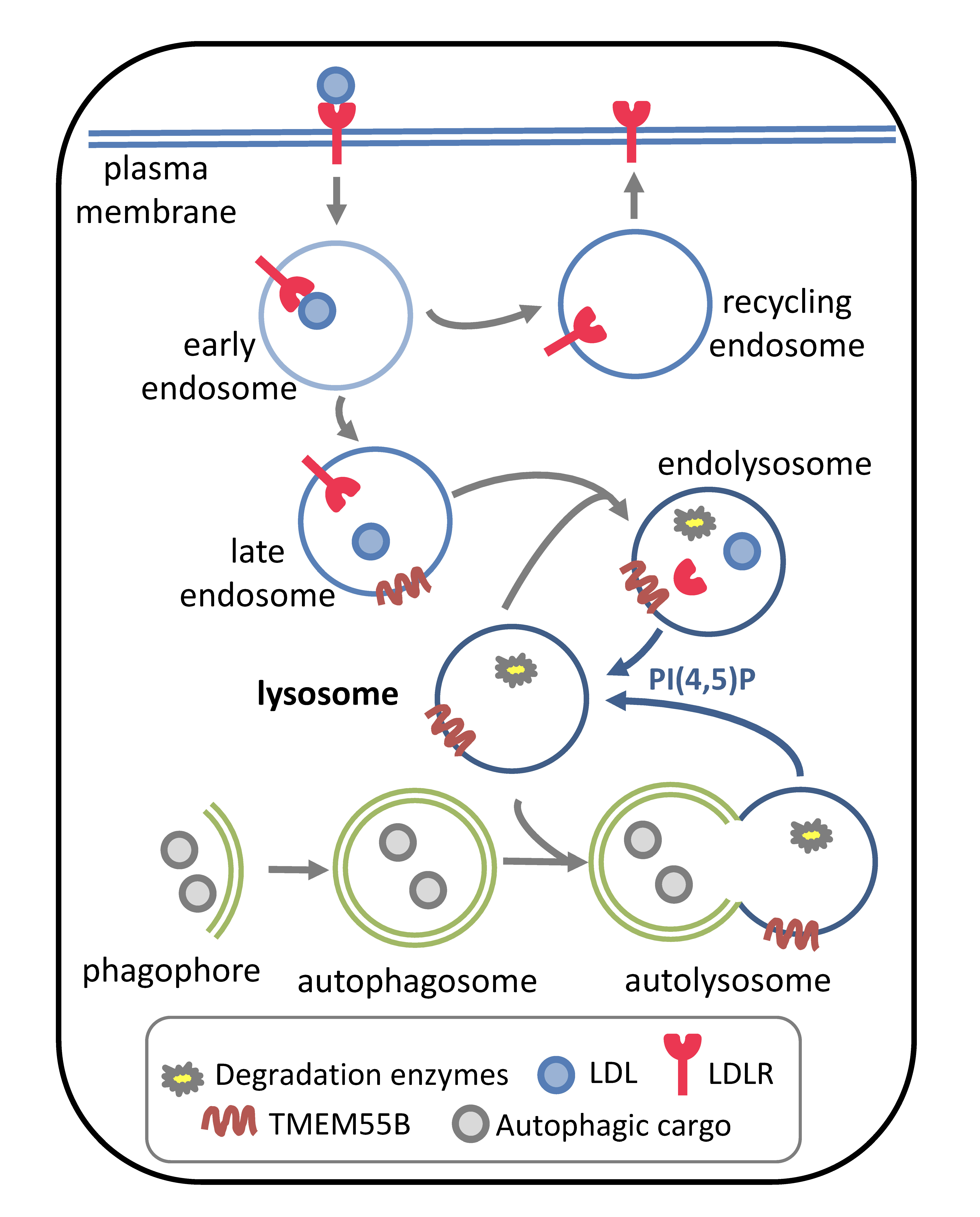
The low-density lipoprotein receptor (LDLR), a major determinant of blood LDL levels, is the most efficacious therapeutic target for the prevention of cardiovascular disease (e.g. statins and PCSK9 inhibitors). In addition, defects in LDLR activity are a primary cause of early-onset cardiovascular disease. Upon LDL binding, LDLR at the plasma membrane is internalized and trafficked to the endosome, where it may be recycled back to the plasma membrane or shunted to the lysosome for decay.
We have reported that transmembrane protein 55B (TMEM55B), a lysosome localized phosphatase that converts phosphatidylinositol (4,5) bisphosphate [PI(4,5)P2] to PI(5)P, stimulates decay of LDLR, impacting LDLR activity and plasma LDL levels. PI(4,5)P2 is an important regulator of lysosome reformation after autophagosome fusion and TMEM55B modulates lysosome levels, localization and activity. We are now evaluating the relationship between TMEM55B and PI(4,5)P2 phosphatases, lysosomal activity and lipid metabolism.
Some images were created using BioRender.
